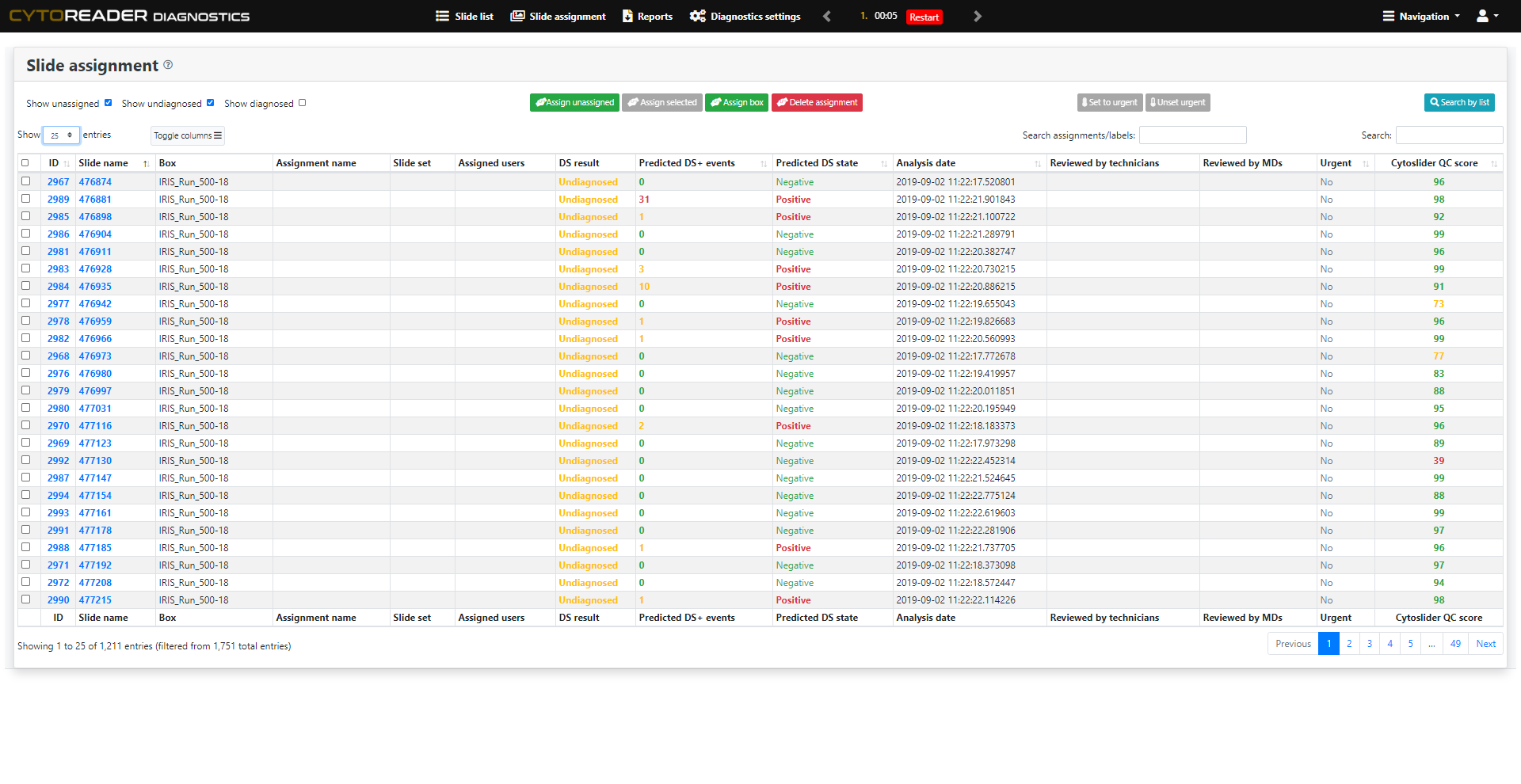
The Challenge & The Solution
Overcoming the Limitations of Manual Dual-Stain Review
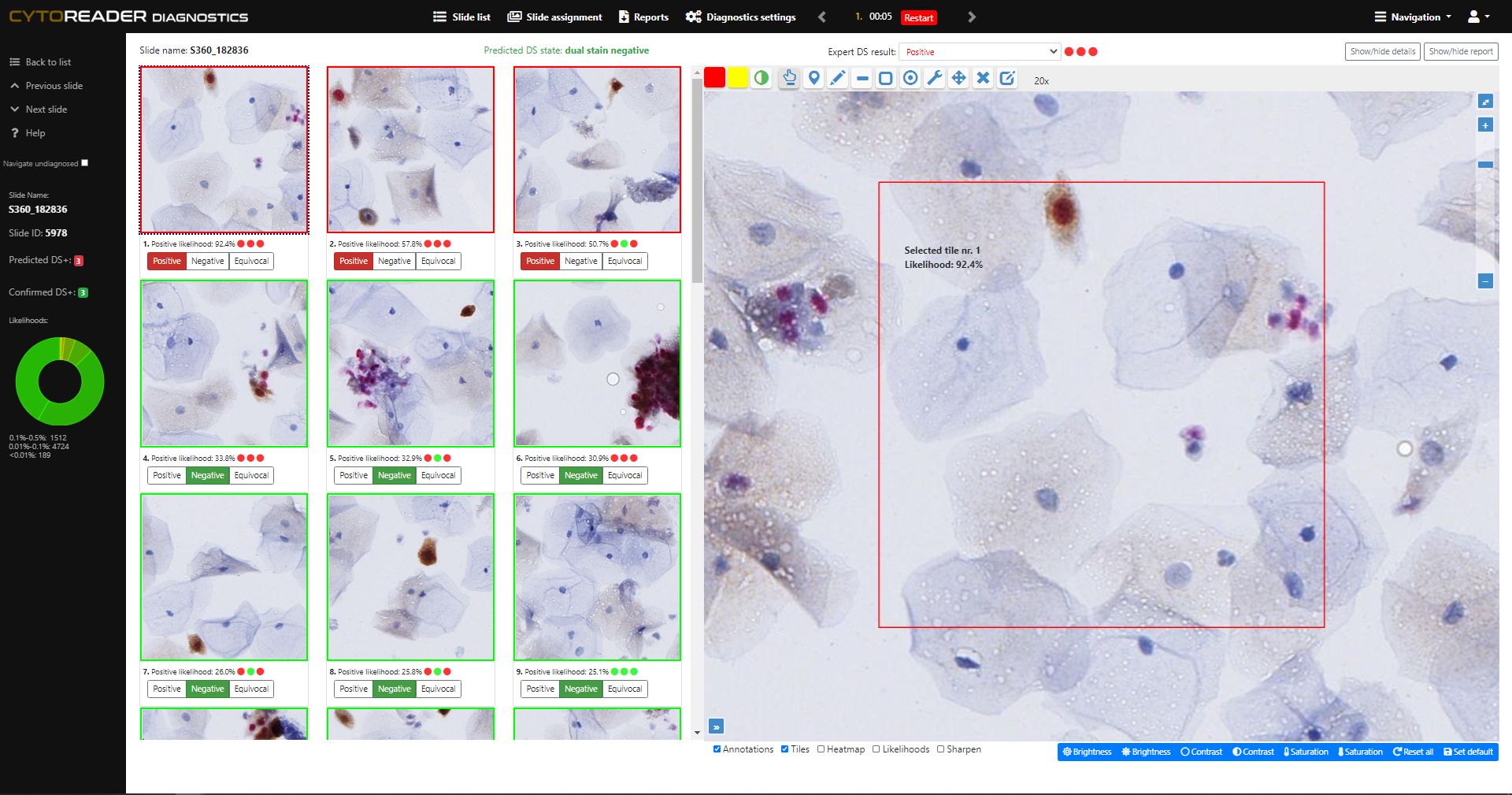
Cervical cancer screening increasingly relies on primary HPV testing, which enables large-scale automation but lacks specificity. p16/Ki-67 dual-stain (DS) cytology is now widely used for triage due to its improved accuracy over Pap cytology—detecting more precancers while reducing unnecessary colposcopies. However, DS brings challenges: manual review is slow, staining varies, and workflows remain analog. Cytoreader tackles these issues using AI and digital technology.:

AI-Assisted Rapid Review
Enables full-slide review in about 10 seconds. The underlying AI algorithm has been shown to reach a performance level comparable to experienced human readers, freeing valuable expert time.

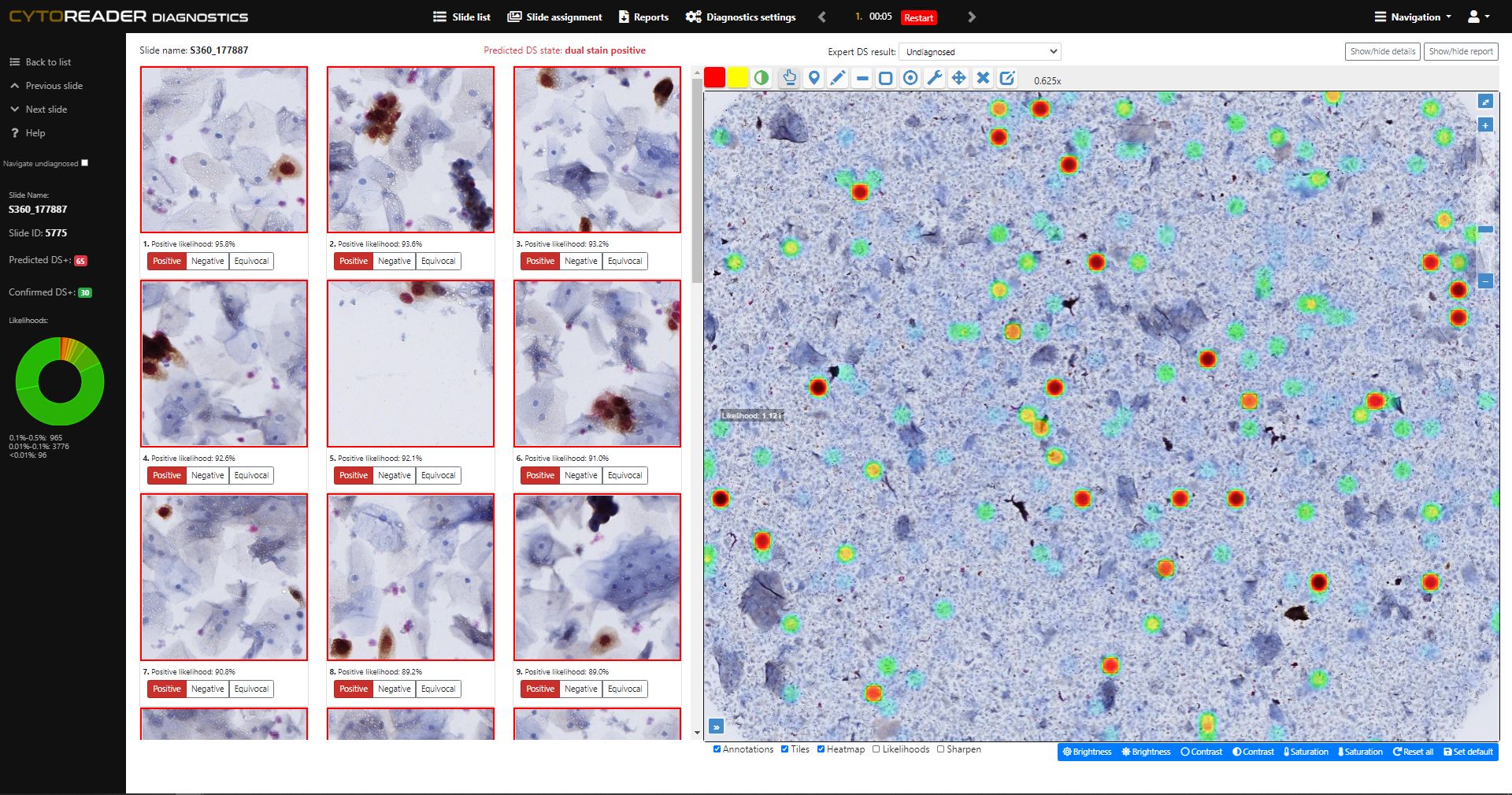
AI-Driven Quality Monitoring
Provides on-slide AI controls that continuously assess staining performance and identify inconsistencies or artifacts early, ensuring reproducible and high-quality workflows.

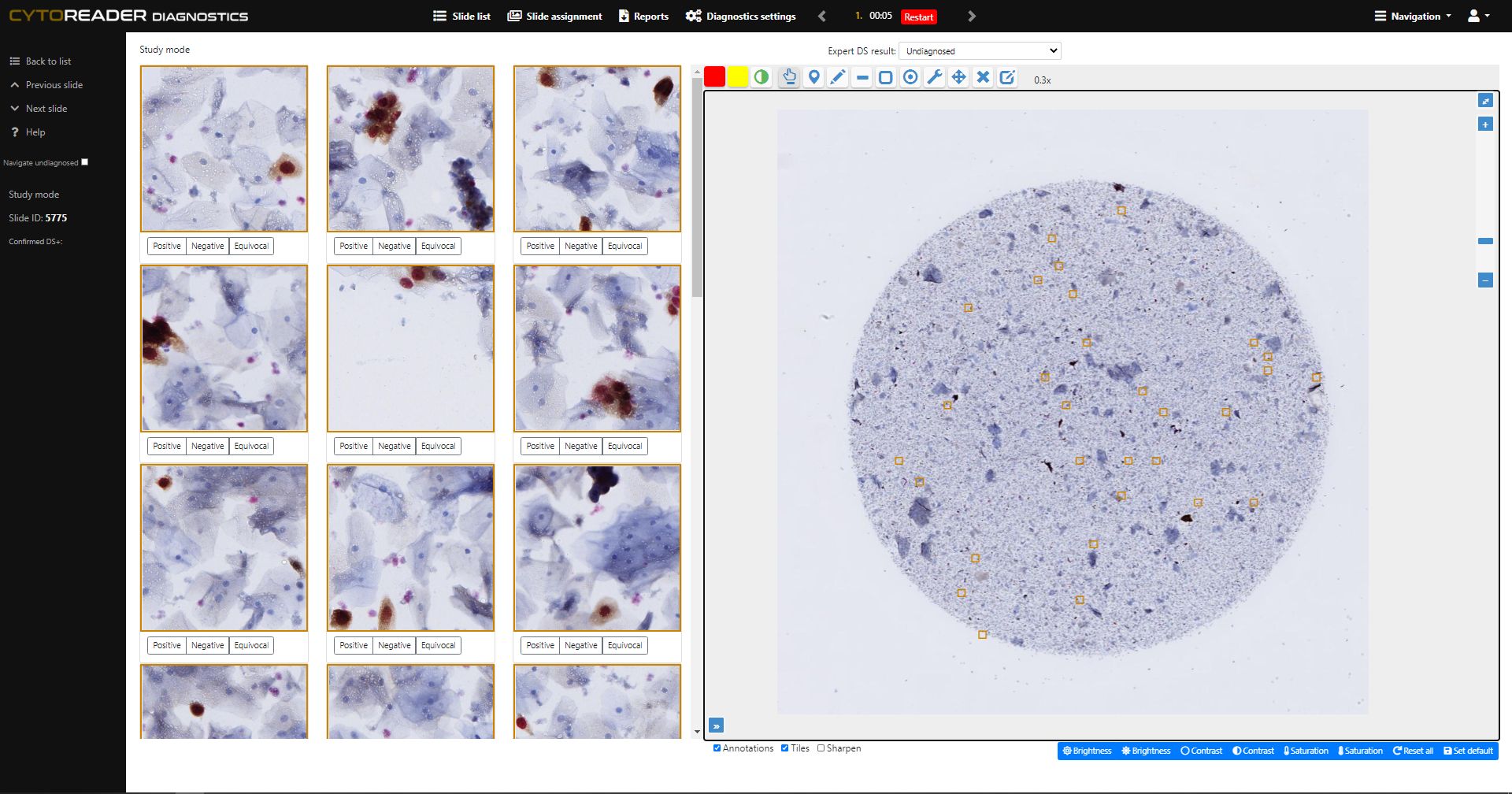
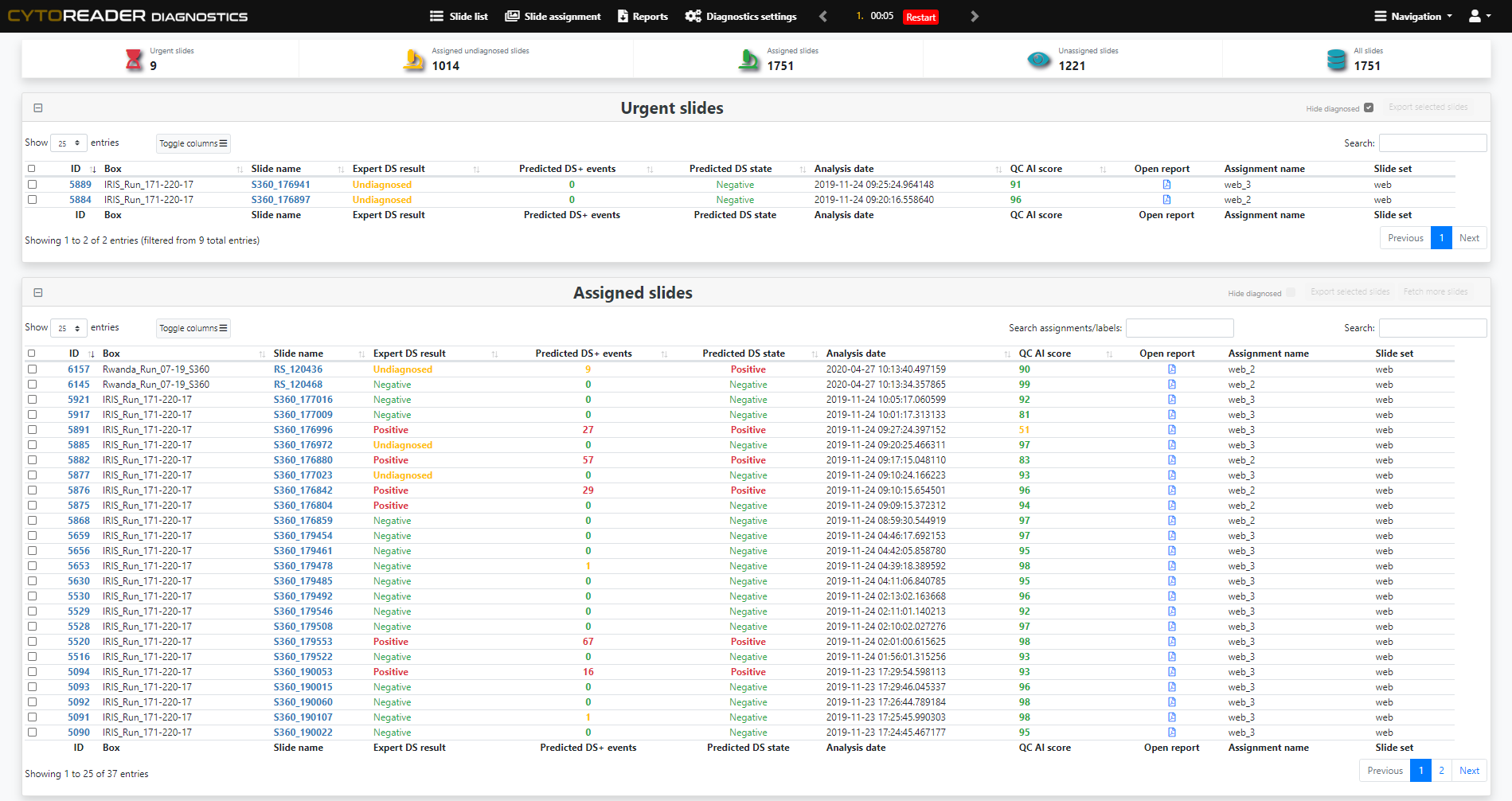
End-to-End Digitalization
Integrates dual-stain slides into a high-throughput digital workflow, including seamless connectivity with Hamamatsu whole slide scanners for ultimate scalability.